Viral Safety
Safeguarding Product Safety: Electron Microscopy for Viral Safety
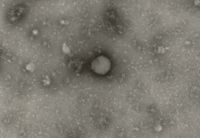
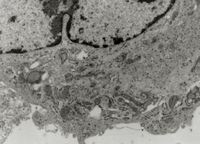
As stated in ICH Q5A (R1 & R2), Transmission Electron Microscopy (TEM) should be performed on the cell substrate utilised in the production of biologics to detect and classify retrovirus-like particles (e.g., type-A and type-C) and on unprocessed bulk harvest samples harvested from bioreactors for the detection and quantification of adventitious agents.
Our team has over 20 years of experience offering validated virus diagnostics and our rapid turnaround times enable prompt identification and resolution of safety concerns, minimizing downtime and accelerating product release.
We offer a full and detailed viral safety assessment of your materials with results issued in under 15 working days.
Test items which are submitted to neotem Bioanalytics consist of either cells or suspended particles in a liquid sample matrix. Accordingly, two separate analytical methods have been established and validated.