Virus Products & Gene Therapy
Unlocking Precision for Viral Products
Our cutting-edge electron microscopy technology offers unparalleled insights into the intricate structures and critical attributes of viral vectors and other viral products.
With our EM-services you can:
🔍 Gain unprecedented clarity: Visualize viral particles in detail, ensuring precise characterization to optimize therapeutic outcomes.
🛡️ Ensure safety: Detect and analyze key quality attributes to guarantee the safety and efficacy of your gene therapy products.
AAV Analysis
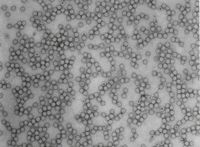
Adeno-Associated viruses (AAV) are one of the go-to vectors for the delivery of therapeutic genes in gene therapy. As gene therapy holds the key for the treatment of a wide variety of diseases, its success stands and falls with the safety and efficacy of the utilised vectors.
We offer a validated and GMP compliant investigation of your AAV particles, focusing on key attributes essential for the optimal performance of your gene therapy vectors:
- Full ratio Determination: Critical for ensuring the right ratio filled particles.
- Particle Morphology: Detailed imaging to confirm structural integrity.
- Particle Integrity: Guaranteeing that particles are intact and functional.
Additional Sample Insights:
- Aggregation Levels and Distribution: To prevent suboptimal dosing or immune responses.
- Process Impurities: Helping you identify and mitigate potential contaminants.
Validation was performed in accordance with ICH Q2 and with thoroughly characterized reference material of different AAV serotypes.
We also investigate and determine the ratio of partially filled particles, incorporating their ratio into the final full:empty determination. Results and Final Reports (CoA) can be obtained in 1-5 working days.