Negative staining: Clarity in shadows
For the detection of adventitious agents in bulk harvest samples and the qualitative and quantitative analysis of suspended particles in liquid sample matrices, we utilise the method of negative staining TEM (nsTEM).
nsTEM:
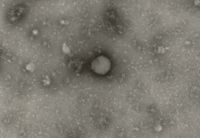
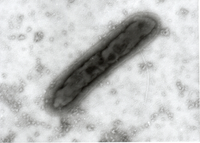
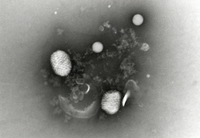
The test item is embedded in a heavy metal salt solution which acts as a contrasting agent deflecting electrons due to its high electron density. As a result the embedded particles appear bright against a dark background when imaged using transmission electron microscopy.
nsTEM is also ideally suited to gain visual feedback on your sample quality in the various steps of upstream and downstream processing (e.g. detect background particles, cell debris, sample impurities, aggregates).
Our state-of-the-art method of QnsTEM for the detection of adventitious agents in unprocessed bulk harvest samples
to enhance the sensitivity, accuracy and robustness of virus detection and quantification in
biological suspensions. QnsTEM eliminates the need to mix beads into the sample by utilizing
reference grids for calibration, thereby reducing variability and improving reproducibility. The
method provides a state of the art and more reliable alternative to existing TEM
based techniques for adventitious agent detection in bulk harvest samples and for the
qualitative investigation of particles. The QnsTEM method enables accurate and precise
quantification of virus particles by transmission electron microscopy using a validated
calibration approach, which has been validated using well characterized reference materials with titers determined by qPCR.